Evaluating the persistence of laundered semen stains on fabric using a forensic light source system, prostate-specific antigen Semiquant test and DNA recovery-profiling
Sukriye Karadayi1 , Elnaz Moshfeghi2, Tulin Arasoglu2 and Beytullah Karadayi3Medicine, Science and the Law 0(0) 1–9The Author(s) 2020 Article reuse guidelines: sagepub.com/journals-permissions DOI: 10.1177/0025802419896935 journals.sagepub.com/home/msl SAGE
Abstract
Semen stains on the clothes of victims of sexual assault can remain as evidence even after garments have been laundered. In this study, we aimed to investigate the effectiveness of commonly preferred methods to detect semen stains in two different fabric types that were laundered with different washing machine programs and washing powders and to obtain a DNA profile from the semen stains. For this purpose, a comprehensive study was performed on semen-stained underwear using three different methods for stain detection, confirmation and identification: a forensic light source (FLS) system, the prostate-specific antigen (PSA) test and DNA recovery profiling. With FLS applications, stronger fluorescence was achieved in wash protocols performed at a low temperature (30_C) on semen-stained cotton under- wear. DNA recovery between 13.45 and 55.00 ng/ml was obtained by modifications in the DNA extraction step when the effect of temperature and washing powder on DNA recovery was evaluated, and these were enough for short tandem repeat (STR) typing in all samples. This study shows that when semen-stained underwear is washed after a month, some semen stains can be determined by FLS and PSA, and all stains can be identified by STR analyses.
Keywords
Laundered semen stains, forensic light source, PSA test, DNA recovery, DNA profiling, STR
Introduction
Sexual violence continues to be a major problem for both children and adults. As a significant number of victims of sexual assault cases do not complain to the judicial authorities,1,2 the prevalence is thought to be even higher than published figures.3 In addition, the number of cases that are not reported because of psychological traumas experienced by the victim is very high.4–6 It was reported in a study evaluating US National Crime Victimization Survey study data that 65% of sexual violence cases were not reported to the police between 2006 and 2010. The results of the same study revealed that sexual crimes constitute the second-highest group of crimes that are not reported after theft crimes.7 Victims of sexual assault are afraid of seeking help for various reasons such as shame and guilt, fear of the perpetrator, social pressure, anxiety that they will not be believed, fear of family members hearing and anxiety of being traumatized again through the judicial process.8–10 In particular, the perpetrators of child sexual abuse can be individuals within the family, as well as individuals outside the family, and these can be the hardest case groups to identify.11Likewise, the cases of female victims living in underdeveloped countries may often remain hidden for long periods because the victims are afraid of social or familial pressures.12–14 As is known, the biological samples considered to belong to the perpetrator are obtained from an examination of the victim’s genital area after the assault, and the investigations on the clothes constitute the most important evidence group.15–17 When there is a delay in the collection of biological evidence after sexual assault, the semen stains on the victim’s clothing can remain detectable for a long time, especially through DNA analysis.18,19In fact, several studies published in recent years have shown that semen stains on clothes can also be detected after laundering.15,20–22 However, further studies are needed to determine the conditions under which the laundered semen-stained clothing can constitute evidence.
Possible semen stains on the victim’s clothing can be determined by using various screening and verification tests in the elucidation of sexual assault cases.15,16,23,24In the next step, DNA is isolated, and subsequently, the DNA profile of the attacker can be determined.15,16,23 In this study, we aimed to investigate the effectiveness of commonly preferred methods in the detection of semen stains on two different fabric types laundered using different washing machine programs and different washing powders and to obtain a DNA profile from the semen stain.
1Vocational School of Health Services, Altınbas ̧ University, Turkey2Department of Molecular Biology and Genetics, Faculty of Arts and Science, Yıldız Technical University, Turkey
Department of Forensic Medicine, Cerrahpasa Medical Faculty, Istanbul University-Cerrahpasa, Turkey
Corresponding author:
Sukriye Karadayi, Vocational School of Health Services, Altınbas ̧ University, 34145, Bakırkoy, Istanbul, Turkey. Email: [email protected]
Methods
Sampling and transfer to fabric procedure
This study consisted of three replicates of 12 different algorithms of different fabric types and washing programs. To create the semen stain, 36 new undergarments were purchased, half of which were cream-colored polyester and half white-colored cotton. The ejaculates were taken from a healthy volunteer donor with a normal sperm count at a specific time interval (at least 48 hours between ejaculations) and stored at +4_C. To determine the location of the semen stains on each undergarment, two circles were drawn with a waterproof pen. To ensure the homogeneity of the stock semen sample, the semen was shaken for about 10 minutes at room temperature and then added to the fabric following pipetting for staining. Each undergarment was stained with 750 ml of semen (72 samples in total). Then, the stained under- wear was dried at room temperature and stored at room temperature for 1 month. After this, they were washed using different washing machine programs and different washing powders. In addition, a biological sample (blood) was taken from the analyst who performed the laundry and all laboratory analyses for control purposes, considering the possibility of contamination. This research was approved by the Ethical Committee of the University of Istanbul University- Cerrahpasa, Cerrahpasa Medical Faculty (REC: 604.01.02-A-36) according to the ethical (Declaration of Helsinki) and legal standards in Turkey.
Laundering protocol
The semen-stained underwear was laundered in a domestic washing machine for 60 minutes using a 1000 rpm program at 30_ C or 60_ C with three different types of washing powder: biological (Sodasan), non-biological (Alomatik) and non-biological washing powder with stain remover added (Kosla). In the washing process, 60 g of washing powder was used each time, but no fabric softener was used. To avoid the possibility of DNA transfer between the underwear, all undergarments were washed separately in the washing machine and dried at room temperature.
Testing for seminal fluids
Each stain created on each undergarment (36 samples) was investigated with a forensic light source (FLS) system to detect the location of the stain. Then, the semen-stained area was cut out and tested for the presence of prostate-specific antigen (PSA) with a SeratecVRPSA Semiquant cassette (cat. no. PSM400F; SeratecVR, Gottingen, Germany). The semen-stained area in the second set of underwear (36 samples) was cut out with sterile scissors and used for short tandem repeat (STR) typing after DNA extraction.
Investigation of seminal fluid using a FLS system (FORENSCOPE).
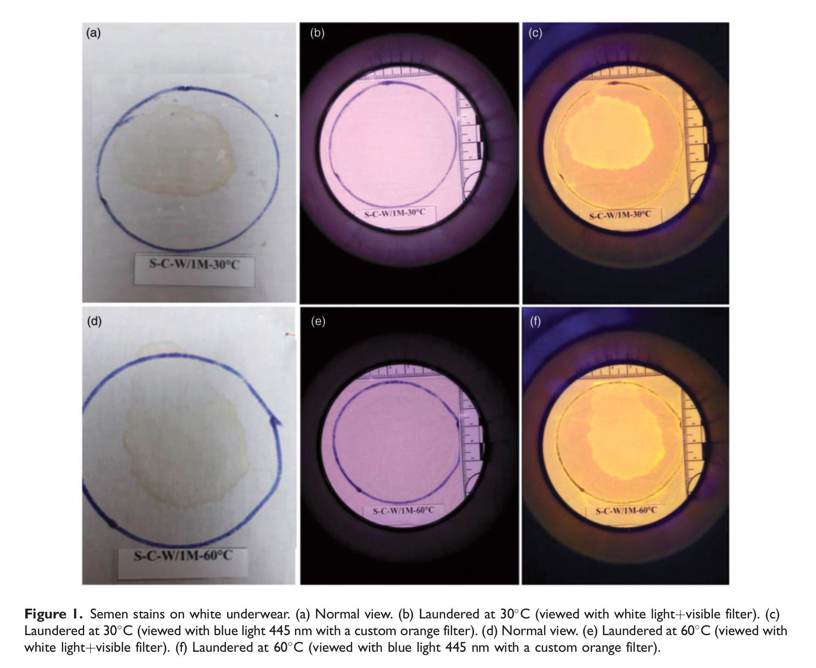
In this study, the detectability of the stain on semen-stained cotton and polyester underwear that was laundered with different washing programs and washing powders was investigated by a FLS system that can provide light at different wavelengths and use different filters with its own imaging. During the investigations, the FORENSCOPE Mobile Multispectral UV- VIS-IR Imaging SystemVR (Grimed Ltd, Voor, The Netherlands) with Android OS was used. This mobile system records with a 13 MP CMOS sensor which is capable of imaging wavelengths of 330–1080_20 nm. The system’s internal camera has a lens with a value of f/2.2 and is capable of continuous autofocus. The system’s camera is integrated into a tablet with Android OS. All the images in the tablet are saved in the forensic software as high-resolution images at a resolution of 4160 _ 3120. Other lights and filter combinations used were as follows: 5.500–6.500 K range white light + visible filter, 360–370 nm range ultraviolet light şcustom yellow filter, 405–425 nm range violet light + custom yellow filter, 440–455 nm range blue light + custom yellow filter, 360–370 nm range ultraviolet light + custom orange filter, 405-425nm range violate light + custom orange filter, and 440-455nm range blue light + custom orange filter. All stains were photographed and saved using the device’s imaging system. To prevent exterior lighting from affecting the images and in order to take the photographs from a standard distance, a specialized dark room headpiece was utilized. The evaluation criteria of the obtained images were defined by the first author as four different categories: negative, weak, moderate and strong.
Immunoassay membrane Semiquant test from semen-stained underwear. The presence of PSA on semen stains was examined with a SeratecVR PSA Semiquant test. According to the manufacturer’s instructions, the cut underwear pieces were kept in 1 ml of buffer solution for two minutes. About 120 ml of this solution was added to the sample well. At the end of the 10-minute incubation period, positive, weak positive and negative results were determined according to the manufacturer’s instructions.
DNA isolation from semen-stained underwear. The semen-stained fabric was cut into 1 cm2 section, placed in a sterile tube and incubated with 2ml of sterile phosphate-buffered saline for 48 hours at room temperature. For DNA isolation, a Quick-DNATMMiniprepPlus kit (Zymo Research, Irvine, CA) was used, with a minor modification to the protocol. In the first stage of DNA isolation, lysis buffer containing 1 M DL-dithiothreitol (anhydrous m.w.1⁄4154.25) was applied instead of proteinase K. Agarose gel electro- phoresis and spectrophotometric (Nanodrop, BioSpec) methods were used for DNA integrity and quality evaluation.
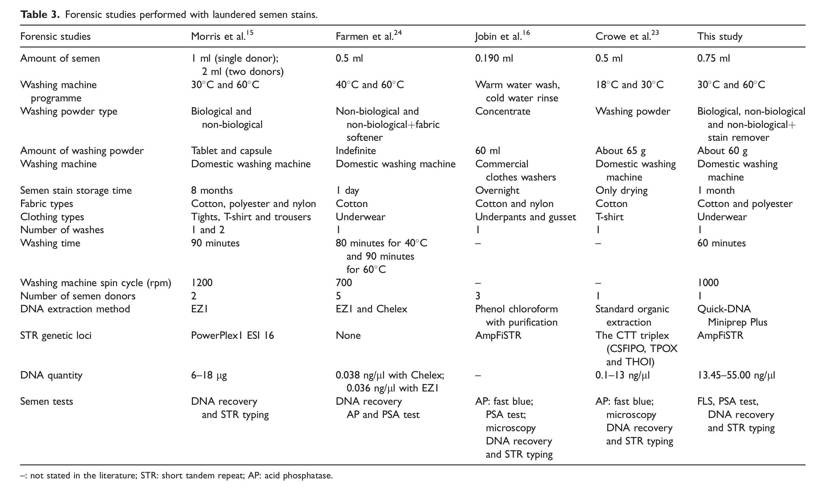
DNA profiling. Each amplification was conducted with 1 ng of DNA using AmpFISTR Identifiler Plus kits (Applied Biosystems, Foster City, CA). STR genetic loci were amplified the AmpFISTR Identifiler Plus PCR Amplification kit (Applied Biosystems) on a 96-well GeneAmp1 PCR System 9700 (Applied Biosystems) for 28 cycles. Amplification products were separated by capillary electrophoresis on an ABI PRISM1 3130xl Genetic Analyser (Applied Biosystems), and data were analyzed using the ABI PRISM1 GeneMapper1 ID software v.3.2 (Applied Biosystems). In this study, we included 15 autosomal STR markers (D8S1179, D21S11, D7S820, CSF1PO, D3S1358, TH01, D13S317, D16S539, D2S1338, D19S433, vWA, TPOX, D18S51, D5S818 and FGA) and Amelogenin (Applied Biosystems AmpFISTR Identifiler Direct User Guide, 2012).
Statistical analyses
Data from three independent studies of all algorithm results generated by washing conditions were analyzed with descriptive statistics using IBM SPSS Statistics v21.0 (IBM Corp., Armonk, NY).
Results
The 72 fabric samples stained on cotton and polyester underwear (light-colored) with semen taken from a single donor were kept at room temperature for 1 month. They were then washed using different washing machine programs and washing powders. After that, the FLS application, PSA test, recovery of DNA and DNA STR typing were used to analyze the fabric samples.
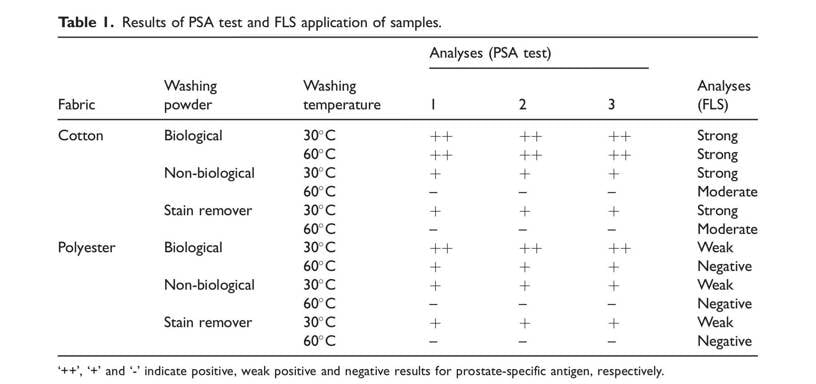
FLS testing
The best images were taken with a 13 MP CMOS sensor, under a 440–455 nm range blue light positioned in a circular orientation and filtered with a custom orange filter specific for the camera. Strong fluorescence was obtained in the examination of the FLS after washing the semen-stained cotton underwear at 30_C. Similarly, strong or moderate fluorescence was observed after washing at 60_C, depending on the washing powder used (Table 1 and Figure 1). In contrast, weak fluorescence was observed after washing the polyester underwear at 30_C, and no fluorescence was obtained in any examinations after washing at 60_C (Table 1).
Immunoassay membrane Semiquant test
Strong positive results were obtained with the PSA Semiquant test after the semen-stained cotton underwear was laundered with the biological washing powder at both temperatures (Table 1). The PSA Semiquant test showed weak positive results after washing cotton underwear at 30_C with both non-biological washing powder and stain remover, but it did not give any positive result after washing at 60_C (Table 1).
Washing the semen-stained polyester underwear with biological washing powder at 30_C resulted in strong positive PSA Semiquant tests. However, when the temperature was increased to 60_C, the results were weak positive. On the other hand, after washing at 60_C with both non-biological powder and stain remover, the PSA Semiquant test result for the polyester underwear was negative.
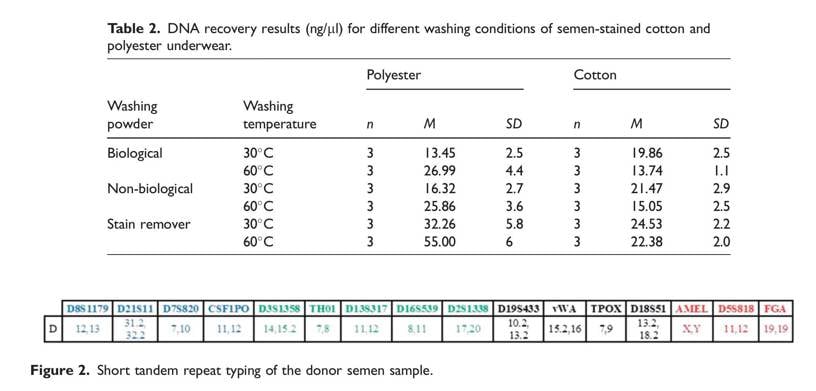
DNA recovery
After laundering all types of semen-stained fabrics, the best DNA recovery results were obtained from the polyester underwear (55.00 ng/ml; Table 2). The DNA recovery studies on semen-stained polyester underwear showed that better results were obtained with the 60_C washing program compared with the 30_C washing program with all types of washing powder. In contrast to this, the DNA recovery for the cotton under- wear was higher when washing at 30_C than when washing at 60_C with all types of washing powders studied (Table 2).
According to the evaluation of DNA recovery according to washing powder type, the best results were obtained with the stain remover, non-biological washing powder, and biological washing powder, respectively (Table 2). When washing the underwear with biological and non-biological washing powder at a low temperature (30_C), the DNA recovery for cotton underwear was found to be higher than that for the polyester fabric. In the DNA recovery studies of underwear laundered at a high temperature (60_C), the result for the polyester underwear was better than for the cotton underwear. When the fabrics were laundered with stain removers at both temperatures, DNA recovery for the polyester fabric was better than for the cotton fabric.
STR typing
The DNA recovery amounts obtained after laundering under different conditions were between 13.45 and 55.00 ng/ml, and these amounts and DNA quality were enough for STR typing. In the DNA isolates obtained from all laundered semen-stained underwear, DNA profiles were obtained that matched the donor and originated from a single source (Figure 2).
Discussion
In many countries throughout the world, after sexual crimes occur, they can be kept hidden for long periods for various reasons, and biological evidence on clothing may not be available. This evidence can be extremely important in the decision-making process of judicial authorities. However, the semen-stained clothes of victims can often be subjected to washing in the period before the case is notified to the judicial authorities. This study aimed to reveal the difficulties that can be encountered in all laboratory stages, from the determination of the stain in the laundered semen-stained underwear to the acquisition of the DNA profile. This study was designed for this purpose with three replicates of 12 algorithms that were designed as the experimental model on different samples formed under the same conditions. In this study, the use of a single donor for experiments and analyses introduced some limitations.
Although the acid phosphatase (AP) test is a frequently used method in detecting semen on stained underwear, in this study, the PSA test was used instead of the AP test because it was reported to give negative results on washed semen stains in the literature.24The PSA Semiquant test that is based on the immunological principle is known to work at 105 dilutions on unwashed semen stains29 and 103 dilutions on washed semen stains.30 The results of this study demonstrated that the laundering temperature and the type of washing powder used to have a significant effect on PSA activity. All the tests conducted after laundering at a low temperature (30_C) were positive for PSA. Similarly, the best PSA test results were obtained after laundering the semen-stained underwear with bio- logical washing powder compared with other types of washing powders. In addition, the PSA tests gave more positive results on laundered cotton underwear compared with laundered polyester underwear (Table 1). Similarly, the study by Jobin and colleagues found that semen stains on the cotton fabric were positive and those on nylon fabric were negative.16 Both semen-stained cotton and polyester underwear gave positive results with the PSA test only after laundering with biological washing powder at 60_C. A laundering temperature of 60_C is thought to be more effective when non-biological washing powder and stain remover are used. Likewise, Laffan and colleagues reported that the PSA test results were weakly positive when garments were laundered at 90_C without awaiting time30(Quick-DNATM MiniprepPlus; Zymo Research).
Microscopic examination results are known to be inadequate when the semen-stained underwear is kept for more than 2 weeks due to the degradation of the sperm cells.24 Thus, direct DNA isolation and STR typing on semen-stained laundered clothes were used in this study. The analyses conducted for this purpose show that the DNA recovery was between 13.45 and 55.00ng/ml with the DNA Extraction Kit (Quick-DNATM MiniprepPlus Kit; Zymo Research). With small modifications in the kit protocol, the DNA amount obtained was increased, and the impurities in the DNA samples were removed. So, DNA recovery was enhanced. Similarly, in the study by Farmen and colleagues which compared different extraction methods on semen-stained fabrics laundered after being kept for 24 hours, an average of 0.038 ng/ml DNA recovery could be obtained with the Chelex method and an average of 0.036 ng/ml of DNA recovery with the EZ1 method.24 On the other hand, in a study conducted by Morris (6–18 mg), where semen-stained fabrics were kept for 8 months, the DNA recovery efficiency was much higher than that of Farmen (0.036 ng/m) and our study (13.45–55 ng/ml).15,24The reason for this is thought to be the storage period of the semen-stained fabrics (Table 3). The reason for the lower recovery of DNA compared with Morris’s study is thought to be related to the storage time of the semen-stained fabrics in this study (Table 3).15,24 As stated in Morris and colleagues’ study, high amounts of DNA recovery were obtained, regardless of washing temperature, washing powder and fabric type.15 This result is also confirmed in the present study. However, when the studies on this subject are compared, it can be seen that there are differences between the results (Table 3).15 Considering the washing temperature, DNA recovery from stained cotton underwear was higher at low temperatures than other temperatures, as indicated in Farmen’s study.24 In contrast to this, a higher yield of DNA recovery from polyester fabrics was achieved at a high temperature (60_ C; Table 2). Similarly, Nussbaumer reported that laundering protocols at high temperatures led to a higher DNA recovery yield and stated that the DNA recovery at 60_C is nearly twice as high as the yield at 40_C.31 However, in the study conducted by Nussbaumer, the type of fabric containing the stain was not specified. It should not be ignored that the difference in DNA recovery between cotton and polyester as related to temperature increase may be caused by the type of fabric as well as the weaving frequency of the fabrics. Therefore, there is a need for studies where the weaving frequency of fabrics is also taken into consideration. In the laundering protocols at the same temperature for different samples in this study, DNA recovery on the cotton samples was achieved at a lower yield by the laundering protocols conducted with biological washing powder compared with non-biological washing powder, as reported in the study by Morris and colleagues.15 However, unlike the work by Morris and colleagues, DNA recovery from polyester underwear was obtained at lower yields with biological washing powder compared with non-biological washing powder. It is thought that this difference may be related to the storage time of the semen stains before washing and to the type of fabric (Table 3).
In terms of stain remover, DNA recovery is more effective for both cotton and polyester underwear than biological and non-biological washing powders. This may mean that the use of stain remover on semen stains is no more effective than washing powder alone. There are many stain removers on the market known to affect different stains. The stain remover used in this study was not very effective at removing semen stains. In addition, this study is the first in the literature where the semen stains were washed with stain removers.
Conclusions
The present study is comprehensive, and it evaluates the process from the determination of stain localization to the individualization stage with the use of different laundered temperatures, different washing powders and different semen-stained fabrics. This study confirmed the knowledge that the amount of DNA recovery obtained from laundered semen-stained clothes varies depending on the type of washing powder, laundering temperature and fabric type. It has been shown that after applying different washing protocols on the formed stains, some semen stains can still be detected by the FLS system and confirmed by the PSA Semiquant test. In addition, a sufficient amount and quality of DNA were achieved for STR analysis in all analyses performed on the stains. Based on the washing temperature, fabric type and detergent type, it is possible to detect semen stains with FLS systems, confirm them with PSA testing and influence the amount of DNA recovery. It is very difficult to locate semen stains with FLS systems on fabrics washed at especially high temperatures. Likewise, it is problematic to obtain results on polyester fabrics with the PSA test. However, an STR profile was obtained from all samples, even those that could not be detected by the screening or confirmation methods.
Acknowledgments
This study was carried out with the support of Istanbul University-Cerrahpasa, Yıldız Technical University and Altınbas ̧ University.
Declaration of conflicting interests
The authors declared no potential conflicts of interest concerning the research, authorship, and/or publication of this article.
ORCID IDs
Sukriye Karadayi: https://orcid.org/0000-0002-4253-9245
Beytullah Karadayı: https://orcid.org/0000-0002-4253-9245 https://orcid.org/0000-0002-1728-0550



